![]()
When starting work on the project, we attempted to list all items produced by the chemical industry for the car industry. Each of us had many suggestions about what we should examine and include into the project. During the work the amount of the accumulated materials grew and we therefore focused our attention on several main topics – fuels, oils, emissions, storage battery. This chapter will therefore be very brief.
Plastics
The extent of use of plastics in the last decades has grown considerably.
The most widely used plastics are:
| Polypropylene | ethylene-propylene rubber | polyurethanes |
| acrylonitrile butadiene styrene | polymethylmethacrylate | polyphenylene oxide |
| polyamides | polycarbonates | polyvinyl chloride |
| polyesters | polyethylene |
Car glass
This is a common sodium-calcium glass. Glass panes are manufactured
by pouring the molten parison on the surface of a tin-containing bath in
a reduction atmosphere. Glass panes of desired dimensions, are then cut
out of the mother pane and shaped in a furnace at 500 – 600 oC.
A layer of organic resin is then applied between two identical glass panes.
Metal materials
Production of metals fall within the field of metallurgy but it is
based on chemical processes. Some metals are currently obtained by hydrometallurgic
processes (e.g. copper), which are energetically more advantageous than
conventional metallurgy. The highest proportion of metal materials is due
to iron - steel and cast iron. A runner-up is aluminium, which is used
in the construction of engines because of its very good heat conductivity.
Aluminium would also be suitable for the car bodies because it is light
and corrosion-resistant (it is passivated by a thin oxide layer). However,
as compared with iron sheets it is less readily shaped and is more expensive.
Zinc is used for the protection of the car body against corrosion (electrochemical
protection). Copper is used for electrical wiring and, in higher-priced
cars, for the cooler construction because its heat conductivity is even
higher than that of aluminium. Lead is part of the storage batteries. Bearings
are fabricated from various metal alloys, which contain Cu, Sn, Pb, Al
and less frequently Sb, Ni, Ag. A current trend is directed at replacing
tin, which is getting rare. Platinum metals are used as catalysts for converting
harmful emission substances into relatively benign ones.
Airbag
An interesting chemical feature in cars is the passive safety element
called airbag. The term is not entirely correct – it should be called "nitrogen
bag". A strong impact will actuate a detonator which in turn triggers a
mechanism shortening the safety belts within 8 ms. Another detonator initiates
a decomposition of sodium or lithium azide, which is placed inside the
airbag. The decomposition proceeds according to the formula
2 NaN3 (s) ® 2 Na (s) + 3 N2 (g)
The reaction is very fast – the bag has to be filled within 30 ms after the impact. The airbag filling also has to contain suitable compounds reacting with the alkali metal formed, probably Al, Si oxides. We calculated how much NaN3 (in grams) would have to be in the airbag filling to fill with nitrogen a bag of 30 l volume at 20 oC and a slight positive pressure of 120 kPa.
Solution:
![]()
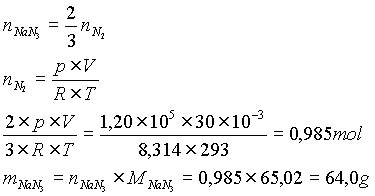
Our survey could include also other chemical industry products such as coating paints, dyestuffs, varnishes, glues, rubber, ceramics, electronics materials, etc. Chemical industry has the extraordinary feature that most of its outputs are used as inputs in other branches and reach the humans only in a "mediated" form.