![]()
The produced fuels have to meet many criteria. In the Kralupy refinery, we saw very well equipped laboratories in which continuous checks of input raw materials and of final output products are carried out. Our guide explained to us, in great detail and in a very interesting way, the principles of individual tests.
We had the chance to see the following methods and instrumentation:
A very important test is the determination of octane number (ON). This test is performed on a special device, which we could see during our second visit to the factory. The final product - gasoline - is mixed from individual components of the gasoline pool so that the required ON is reached. The refinery produced leaded gasolines - Special 91, Super 96, and two types of unleaded gasolines, Natural 95 and Natural 98.
The octane number
is equal to such a volume per cent of 2,2,4-trimethylpentane (iso-octane,
whose ON is 100) in a mixture with heptane (ON 0), which is needed to achieve
the same mixture quality as the evaluated gasoline.
The principle of the assay is the determination of the engine advance at which the engine begins to "knock" due to spontaneous detonations during the combustion process. The knocking is determined by detonation sensor. The engines in the laboratory are one-cylinder, with variable combustion space volume. Electrical engine is used to start up the engine and to keep its revolutions. The standardisation fuel is a mixture of iso-octane and n-heptane in a certain ratio. The octane number of the gasoline has to lie between the values for two standard fuels differing by 1 octane unit. The measurement is repeated several times and the results are averaged. The laboratory employs two methods, a research and a motor one, which provide two octane number values (RON - research octane number, and MON - motor octane number). The number shown at petrol stations is always RON. Sometimes the RON of a gasoline is satisfactory but the MON is not.
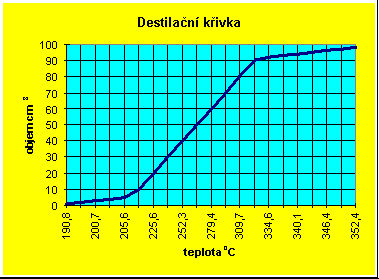
Distillation curve
We measured the curve ourselves during our second visit to the Fuel
and Lubricant Research Institute. The record of the test is enclosed (see
page 17). Our sample was Diesel oil. A volume of 100 cm3 is
placed into a distillation flask and the flask is inserted into the apparatus
in which its bottom is electrically heated. The vapour in pipes passes
through a cooling bath whose initial temperature is 46 oC (0
oC for gasolines). The distillate volume in cm3 (column
1 left) and the corresponding boiling point (column 2) are recorded. The
initial value in column 3 is 59 s - the time from the beginning of heating
to the appearance of the first distillate drop. If the time is 50 - 70
s, i.e. if it corresponds to a standard, the sample is satisfactory. Other
data in the column show the velocity of distillation in per cent of the
original sample volume per min. The velocity of distillation is seen to
decrease gradually, corresponding to the distillation of higher-boiling
components. The total distillate volume was 98.7 cm3, the losses
amounted to 1.3 %.
The course of the curve shows readily if the gasoline has been polluted with Diesel oil or vice versa - this may easily happen during the exchange of fuels in transport.
Our visit to the Department of Oil Technology and Petrochemistry of the Institute of Chemical Technology included observation of the work with gas chromatograph, with a capillary column and a FID detector. The injected gasoline sample was 0.1 m l. After gassification the sample was diluted 1:200 and applied on the column. The resulting chromatogram is enclosed.
Then we had the opportunity to become acquainted with a GC-MS apparatus,
which combines gas chromatograph with mass spectrometer. A chromatogram
was displayed on the screen of a computer connected with the apparatus.
Each of us could then choose arbitrarily a peak; the computer showed the
corresponding mass spectrum and compared it with a spectra library. The
display then showed the substances, which could correspond to the spectrum,
listed according to the degree of spectra identity. In the laboratories,
we also examined an HPLC liquid chromatograph, which is used for analysis
of polyaromatic hydrocarbons in emissions. For this analysis, fluorimetric
detector is the most suitable.