![]()
![]() To become acquainted with the production of fuels, we visited the Czech Refineries (Česká rafinérská a.s.) enterprise in Kralupy nad Vltavou. It is a fuel refinery specialising in the production of engine fuels and heating oils. We visited the control centre and the very well equipped laboratories. During a second visit we paid most attention to the measurement of gasoline octane numbers. The enterprise heavily invests into new technologies. Since April 1997, a new unit for C5, C6 fraction isomerisation has been operating here. In the near future the construction will start of a fluid catalytic cracking (FCC) unit which should be finished in 2001. These investments are vitally important for the enterprise. The refinery processes about 32 million tonnes of oil annually. It is located next to the Kaučuk enterprise, which uses the C4 fraction for the production of butadiene. The by-product of this production is butenes, which are used in the production of methyltert. butylether (MTBE). This compound is added (up to 10 %) to the gasolines produced in the refinery. The gasolines are currently produced in a catalytic reforming unit.
To become acquainted with the production of fuels, we visited the Czech Refineries (Česká rafinérská a.s.) enterprise in Kralupy nad Vltavou. It is a fuel refinery specialising in the production of engine fuels and heating oils. We visited the control centre and the very well equipped laboratories. During a second visit we paid most attention to the measurement of gasoline octane numbers. The enterprise heavily invests into new technologies. Since April 1997, a new unit for C5, C6 fraction isomerisation has been operating here. In the near future the construction will start of a fluid catalytic cracking (FCC) unit which should be finished in 2001. These investments are vitally important for the enterprise. The refinery processes about 32 million tonnes of oil annually. It is located next to the Kaučuk enterprise, which uses the C4 fraction for the production of butadiene. The by-product of this production is butenes, which are used in the production of methyltert. butylether (MTBE). This compound is added (up to 10 %) to the gasolines produced in the refinery. The gasolines are currently produced in a catalytic reforming unit.
![]() Crude oil began to be processed in the 19th century, approximately from 1820. The main product at that time was kerosene for lamps, later lubrication oils. Following the invention of gasoline engines, engine fuels became the most important products, to be gradually supplemented by heating oils. Today, about 25 % of oil production is processed to engine fuels and about 50 % to heating oils. Other products obtained from oil are asphalt, lubricating oils, paraffin, and gas fuels and petrochemical raw materials.
Crude oil began to be processed in the 19th century, approximately from 1820. The main product at that time was kerosene for lamps, later lubrication oils. Following the invention of gasoline engines, engine fuels became the most important products, to be gradually supplemented by heating oils. Today, about 25 % of oil production is processed to engine fuels and about 50 % to heating oils. Other products obtained from oil are asphalt, lubricating oils, paraffin, and gas fuels and petrochemical raw materials.
![]() Oils from different location can considerably differ in their density, colour, types of hydrocarbons and the content of non-hydrocarbon compounds. The basis is formed by C5-C35 hydrocarbons (plus small amounts of higher hydrocarbons). Dissolved gaseous C1-C4 alkanes are also present. Some oils contain substantial proportions of so-called oil resins and asphaltenes (Mr = 2000 - 3000) which impart to oil its dark colour and are converted to the macromolecular aphalts during processing.
Oils from different location can considerably differ in their density, colour, types of hydrocarbons and the content of non-hydrocarbon compounds. The basis is formed by C5-C35 hydrocarbons (plus small amounts of higher hydrocarbons). Dissolved gaseous C1-C4 alkanes are also present. Some oils contain substantial proportions of so-called oil resins and asphaltenes (Mr = 2000 - 3000) which impart to oil its dark colour and are converted to the macromolecular aphalts during processing.
![]() The Kralupy refinery is supplied by oil via two oil pipelines, one from Russia and the other (since 1995) from Ingoldstadt.
The Kralupy refinery is supplied by oil via two oil pipelines, one from Russia and the other (since 1995) from Ingoldstadt.
![]() Crude oil contains a small proportion of emulsified water with dissolved salts. This fraction is removed by sedimentation after the addition of de-emulsifiers. The resulting oil is then preheated in a tube furnace and is injected into a large atmospheric-pressure column. Three fractions are drawn off from the upper part of the column, the distillation residue being mazut.
Crude oil contains a small proportion of emulsified water with dissolved salts. This fraction is removed by sedimentation after the addition of de-emulsifiers. The resulting oil is then preheated in a tube furnace and is injected into a large atmospheric-pressure column. Three fractions are drawn off from the upper part of the column, the distillation residue being mazut.
![]() All these fractions are conveyed to hydrogenation refining. The hydrogenation takes place at a pressure of about 3 MPa, the catalyst contains molybdenum and cobalt. The hydrogenation involves the following transfer reactions:
All these fractions are conveyed to hydrogenation refining. The hydrogenation takes place at a pressure of about 3 MPa, the catalyst contains molybdenum and cobalt. The hydrogenation involves the following transfer reactions:
| O ® H2O | S ® H2S | N ® NH3 |
![]() H2S is washed with diethanolamine and further processed to sulphur. This sulphur is supplied to the near chemical enterprise Spolana Neratovice to be used for the production of H2SO4. Fraction 1 is redistilled into three fractions with boiling points up to 65 oC, up to 85 oC and up to 175 oC. The lightest C5, C6 fraction is then taken to low-temperature isomerisation. The isomerisation takes place at 120 - 150 oC, the catalyst being platinum on acid alumina (pH 0 -1). Another type of catalyst for this process is zeolites. Pentane is converted to isopentane, hexane to 2,2-dimethylbutane. The objective of the process is to enhance the octane number (ON).
H2S is washed with diethanolamine and further processed to sulphur. This sulphur is supplied to the near chemical enterprise Spolana Neratovice to be used for the production of H2SO4. Fraction 1 is redistilled into three fractions with boiling points up to 65 oC, up to 85 oC and up to 175 oC. The lightest C5, C6 fraction is then taken to low-temperature isomerisation. The isomerisation takes place at 120 - 150 oC, the catalyst being platinum on acid alumina (pH 0 -1). Another type of catalyst for this process is zeolites. Pentane is converted to isopentane, hexane to 2,2-dimethylbutane. The objective of the process is to enhance the octane number (ON).
![]() Heavy gasoline (b.p. 100 - 200 oC) is taken to catalytic reforming. This process yields a high-octane gasoline from a low-octane one, and also hydrogen which is then used for hydrogenation refining. What reactions take place here?
Heavy gasoline (b.p. 100 - 200 oC) is taken to catalytic reforming. This process yields a high-octane gasoline from a low-octane one, and also hydrogen which is then used for hydrogenation refining. What reactions take place here?
![]() The major reaction is hydrogenation (the process is thus endothermic) and isomerisation of alkanes to isoalkanes. The reactions take place at about 500 oC and a pressure of 1.5 MPa in the presence of a catalyst - a Pt - Rh system on acid alumina. An example of such a reaction is:
The major reaction is hydrogenation (the process is thus endothermic) and isomerisation of alkanes to isoalkanes. The reactions take place at about 500 oC and a pressure of 1.5 MPa in the presence of a catalyst - a Pt - Rh system on acid alumina. An example of such a reaction is:
![]()
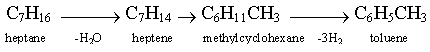
![]() It is clear that this process yields large amounts of aromatics, which strongly increase the octane value. However, the current trend is directed towards lowering the content of aromatics, especially benzene.
It is clear that this process yields large amounts of aromatics, which strongly increase the octane value. However, the current trend is directed towards lowering the content of aromatics, especially benzene.
![]() The refinery now obtains gasoline from 20 % of the processed oil. The demand for gasolines rises and it is thus necessary to use the heavier, higher-boiling-point oil fractions. These higher hydrocarbons have to be cleaved or cracked to lower ones. This is achieved in a process called cracking. The oldest technology is thermal cracking (as early as after 1910) which is nowadays used only to a small extent. It affords low gasoline yields with low ON and low stability (in view of the alkene content).
The refinery now obtains gasoline from 20 % of the processed oil. The demand for gasolines rises and it is thus necessary to use the heavier, higher-boiling-point oil fractions. These higher hydrocarbons have to be cleaved or cracked to lower ones. This is achieved in a process called cracking. The oldest technology is thermal cracking (as early as after 1910) which is nowadays used only to a small extent. It affords low gasoline yields with low ON and low stability (in view of the alkene content).
![]() A modern technology which is to be soon set up in the refinery is fluid catalytic cracking (FCC). This cracking takes place in a fluid bed at 510 oC, a pressure of 0.2 - 0.3 MPa, with zeolites as catalysts. Apart from gasoline, its important products are light C3 - C5 alkenes which can be used for alkylation or in the production of MTBE and TAME (tert. amylmethylether).
A modern technology which is to be soon set up in the refinery is fluid catalytic cracking (FCC). This cracking takes place in a fluid bed at 510 oC, a pressure of 0.2 - 0.3 MPa, with zeolites as catalysts. Apart from gasoline, its important products are light C3 - C5 alkenes which can be used for alkylation or in the production of MTBE and TAME (tert. amylmethylether).
![]() A promising technology, which is to be considered for future use, is alkylation. Isobutane from FCC could be alkylated by C3 - C5 alkenes. The alkylation would proceed at low temperatures (below 40 oC) and moderate pressures, 0.1 - 1 MPa, with strong acid acting as catalyst. The alkylation product - alkylate - has a high ON and low volatility. The near future is certain to see the advent of so-called reformulated gasolines, i.e. gasolines which meet the strictest demands for emission production - low volatility, low content of aromatics, especially benzene, very low sulphur content and the presence of oxygen-containing components (the presence of oxygen in the fuel contributes to better combustion but the energy content of the fuel is somewhat lower). The oxygen-containing components are mostly ethers - MTBE, TAME.
A promising technology, which is to be considered for future use, is alkylation. Isobutane from FCC could be alkylated by C3 - C5 alkenes. The alkylation would proceed at low temperatures (below 40 oC) and moderate pressures, 0.1 - 1 MPa, with strong acid acting as catalyst. The alkylation product - alkylate - has a high ON and low volatility. The near future is certain to see the advent of so-called reformulated gasolines, i.e. gasolines which meet the strictest demands for emission production - low volatility, low content of aromatics, especially benzene, very low sulphur content and the presence of oxygen-containing components (the presence of oxygen in the fuel contributes to better combustion but the energy content of the fuel is somewhat lower). The oxygen-containing components are mostly ethers - MTBE, TAME.
![]() All components available at the refinery form the so-called gasoline pool.
All components available at the refinery form the so-called gasoline pool.
They include:
Diesel oil is used as fuel for compression-ignition engines. It is basically a mixture of fraction 2 and fraction 3(b.p.170 - 370oC) from primary oil distillation (C12 - C18) and a gas oil (C15 - C24). With this fuel, the demands for fuel processing are substantially lower, and involve mostly removal of sulphur. Fuel combustion occurs at higher temperatures and the composition of emissions is therefore different from that in spark-ignition engines. In terms of demands for fuel quality and of emissions, compression-ignition engines thus appear to be more advantageous that spark-ignition ones, but they are much more robust and expensive in view of the high working pressures.
The refinery supplies oils to the Prague Ruzyně
airport. Aircraft gasoline is a fraction with boiling points in the range
150 - 280 oC. It has to be processed so that its viscosity
at low temperatures would not be too high. It must not solidify up to -60
oC.
Fuels produced from renewable resources, e.g. ethanol produced by fermentation, offer other possibilities. The so-called bio-gasoline contains methyl esters of higher fatty acids. They are produced from lower-quality plant oils.
A promising way is the use of hydrogen, which can be produced by different methods - electrolytically and chemically - from water and can be transported in a liquefied form or dissolved in metal alloys. Its advantages as a car fuel are:
For this reason, better alternative energy sources would be fuel cells in which a direct conversion of chemical energy to work takes place. Hence, their efficiency is not limited by the second law of thermodynamics and is about twice as high as in combustion engines (about 70 %). The best known is probably the hydrogen-oxygen cell, which may have, for instance, the following arrangement:
Anode - oxidation: 2 H2 (g) + 4 OH- (aq) ®
4 H2O (l) + 4 e-
Cathode - reduction: O2 (g) + 2 H2O (l) + 4 e-
® 4 OH- (aq)
The overall reaction is the sum of partial electrode reactions: 2H2(g) + O2 (g) ® 2 H2O (l).
Hydrogen is continuously fed to the anode while oxygen is brought to the cathode. The KOH solution is kept hot, water vapour escapes from the electrode space. Ni and NiO function as electro-catalysts. Other fuel cells have been developed which are capable of "burning" hydrocarbons in the cold. For instance, the propane-oxygen cell:
Anode: C3H8 (g) + 6 H2O (g) ®
3 CO2 (g) + 20 H+ (aq) + 20 e-
Cathode: 5 O2 (g) + 20 H+ (aq) + 20 e-
® 10 H2O (l)
Overall reaction: C3H8 (g) + 5 O2 (g) ® 3 CO2(g) + 4 H2O(l)
In contrast to storage batteries, fuel cells cannot store energy. The reactants have to be continuously supplied and the products continuously removed. Fuel cells do not produce waste heat, vibrations, noise, and toxic substances. Despite these merits, they are not used to a large extent. The main problem is the selection of suitable electro-catalysts, which would exhibit long-term resistance to contamination and corrosion.
A very promising energy source is the system illustrated in the figure.
It is basically a combination of a galvanic and a fuel cell.
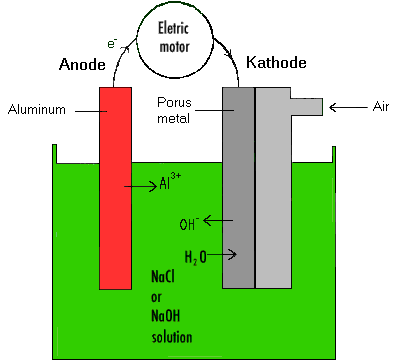
Electromobiles are certain to experience widespread use in the future, albeit for shorter cruises at lower speeds. On a mass scale, however, the transports will still use gasoline and Diesel oil. The American companies Ford and Chrysler have developed a hybrid automobile equipped with storage batteries and electric engine and, in addition, a small combustion engine. This automobile should reach a speed of up to 160 km.h-1 at a presumed fuel consumption of 2.6 l/100 km.