![]()
To learn more about automobile fuels and emissions, we visited the Department of Oil Technology and Petrochemistry, which constitutes part of the Faculty of Environmental Protection. This Faculty is one of the four faculties of the Institute of Chemical Technology in Prague. The Institute closely co-operates with oil refineries, with car industry and with other research institutes. One of the major research topics is the relationship between the fuel composition and the emissions, with special reference to polyaromatic hydrocarbons and aldehydes, i.e. components for which emission limits have as yet not been established. We learned many interesting things and obtained copies of research reports and scientific papers, which we distributed among ourselves for study.
Few of us realize that the atmosphere is polluted with hydrocarbons (HC) as a result of vaporisation of gasoline from the fuel system of cars, both when they move and stand still, and during fuelling. This pollution corresponds to the fuel composition and the volatility of its components, and rises naturally with increasing temperature. The ratio of the amount of HC in exhaust gases to the amount of HC released into the air due to vaporisation is about 5:3.
Measures designed to lower these emissions
- The refineries reduce the content of the most volatile HC, i.e. the
C4-fraction. These components have in the past been used as
high-octane-number fractions of the gasoline pool.
- New cars are routinely equipped (in western Europe since 1 January 1993) with auxiliary charcoal tanks, the so-called Carbon Canisters (CC) in which the vaporised HC are first adsorbed and, in a recycling phase, are desorbed at elevated temperature into the suction system of the running engine. If the adsorption capacity of the device is sufficient, as in the so-called Large Carbon Canisters (LCC), it removes efficiently also the fuel vapours released during fuelling (about 1.3 g gasoline per litre). The local concentrations of HC around the gasoline station gas pumps are very high and can be dangerous for the health of the service personnel.
Main components: CO2, H2O (g), N2 - relatively harmless
Harmful components:
a) Components whose content is set by law, i.e. CO, NOx,
HC, sometimes HCHO. The term hydrocarbons covers both uncombusted hydrocarbons
from the fuel and hydrocarbons newly formed during the combustion process.
b) Components whose content is not yet defined by law but are under investigation because of their harmful effect on living organisms. These are particularly benzene, 1,3-butadiene, polyaromatic hydrocarbons (PAH), nitrated PAH (N-PAH), formaldehyde and acetaldehyde. The levels of PAH and N-PAH are very low (and their determination is therefore exacting and complicated) but their health risk degree is very high. Legal limits to the contents of some of these substances can also be expected to be set in the near future.
c) In leaded gasolines, an additional emission component is lead in various forms. Addition of alkylhalogenides as Pb carriers is currently being abandoned; consequently, lead, which used to be released into environment as PbCl2 or PbBr2, is now present in other forms - PbO, Pb3O4 or sometimes PbSO4 because the fuel contains also a very low percentage of sulphur (below 0.005%). These forms are less volatile and less soluble.
For illustration of the variegated composition of car exhaust emissions, we give a survey of compounds identified in the analysis of volatile HC (Table 1) and a survey of identified PAU (Table 2) emitted by spark-ignition engines. The data are taken over from the research report "Elaboration of methodology for determining organic substances in exhaust gases from car gasoline combustion", Department of Oil Technology and Petrochemistry, Institute of Chemical Technology, Prague.
Composition of exhaust gases from compression-ignition engines
The emission of basic harmful pollutants (see under a) is substantially
lower but this fact is offset by a higher particulate pollution constituted
especially by carbon black, which serves as sorbent carrier for the highly
harmful PAH. Moreover, the fuel contains a non-negligible level of sulphur
(European Standard EN 590 from 1/10/1996 permits a maximum of 0.05 %) which
is emitted as SO2.

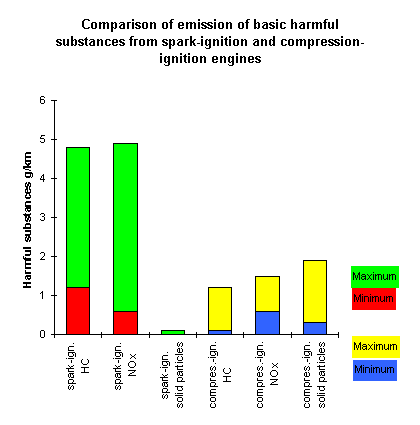
Comparison of minimum and maximum emission levels of basic
harmful substances from spark-ignition and compression-ignition engines.
The range between the minimum and the maximum covers the differences between
different engine types or sizes.
n-Alkanes
| propane | butane | pentane | hexane | heptane | oktane | nonane |
i-Alkanes
| 2-methylpropane | 2-methylbutane | 2,3-dimethylbutane | 2-methylpentane | 3-methylpentane |
| 2,2-dimethylpentane | 2,4-dimethylpentane | 3,3-dimethylpentane | 3-ethylpentane | 3-methylhexane |
| 2-methylhexane | 2,2-dimethylhexane | 2,5-dimethylhexane | 2,4-dimethylhexane | 3,3-dimethylhexane |
| 2,3-dimethylhexane | 2-methylheptane | 4-methylheptane | 3,4-dimethylhexane | 3-methylheptane |
| 2,2-dimethylheptane | 2,4-dimethylheptane | 3,3-dimethylheptane | 4-ethylheptane | 4-methyloktane |
| 2-methyloktane | 3-ethylheptane | 3-methyloktane | C10-isoalkanes | C11- isoalkanes |
| Cyklopentane | Methylcyklopentane | cyklohexane | 1,1-dimethylcyklopentane |
| 1,3-dimethylcyklopentane | 1,2-dimethylcyklopentane | methylcyklohexane | 1,1,3-trimethylcyklopentane |
| Ethylcyklopentane | 1,2,4-trimethylcyklopent. | 1,2,3-trimethylcyklopent. | 1,1,2-trimethylcyklopentane |
| 1,3-dimethylcyklohexane | 1,4-dimethylcyklohexane | 1,1-dimethylcyklohexane | 3-ethyl-1- methylcyklopent. |
| 2-ethyl-1-methylcyklopent | 1-ethyl-1-methylcyklopent | 1,2-dimethylcyklohexane | 1,1,4-trimethylcyklohexane |
| Alkylcyklopentanes C9 -C11 | alkylcyklohexanes C9 -C11 |
Aromates
| Benzene | Toluene | m-xylene | p-xylene |
| o-xylene | i-propylbenzene | propylbenzene | 1-ethyl-3-methylbenzene |
| 1-ethyl-4-methylbenzene | 1,3,5-trimethylbenzene | 1-ethyl-2-methylbenzene | 1,2,4-trimethylbenzene |
| i-butylbenzene | 1,2,3-trimethylbenzene | indane | methylindane |
| C4-alkylbenzenes | C5-alkylbenzenes | naftalene | 1-methylnaftalene |
| 2-methylnaftalene |
Alkenes
| 1-butene | 2-butene | 2-methylpropene | 3-methyl-1-butene | 1-pentene |
| 2-methyl-1-butene | 2-pentene | 2-methyl-2-butene | cyklopentene | 4-methyl-1-pentene |
| 3-methyl-1-pentene | 2-methyl-1-pentene | 1-hexene | 3-hexene | 2-hexene |
| 2-methyl-2-pentene | 3-methyl-2-pentene | dimethylpentenes | methylhexenes | ethylpentene |
| Styrene | methylstyrene |
Oxycompounds
methyl-t-butylethere
| Fluorene | C1 - C2 alkylfluorenes | Fenanthrene | C1 - C4 fenanthrenes |
| Anthracene | C1 - C2 alkylanthracenes | Fluoranthene | methylfluoranthenes |
| Pyrene | C1 - C3 alkylpyrenes | Chrysene | benzo[ b] fluoranthene |
| benzo[k]fluoranthene | benzo[ e] pyrene | benzo[ a] pyrene | benzo[ ghi] perylene |
| Dibenzo[ e,l] pyrene | koronene |
We should like to conclude this survey of the emission composition by pointing out a fact that is rarely considered. The combustion of any fuel consumes oxygen and gives rise to the non-toxic carbon dioxide. The content of CO2 in the atmosphere dangerously increases and, along with other gases, CO2 is known to cause the so-called greenhouse effect. The scientists are not yet fully agreed on whether a global warming up will occur which would have disastrous consequences for mankind. At any rate, it is clear that the natural balance has been disturbed owing to combustion processes. The importance of the problem is attested also by the fact that it was the subject of an international conference on CO2 pollution in Kyoto, Japan, in December 1997.
A simple calculation, in which we will neglect the incompleteness of the combustion and will not take into account the content of oxygen-containing components of the fuel, will show how much oxygen automobiles consume and how much CO2 they produce.
Example:
To cover a distance of 100 km, a car consumes 7.0 dm3 gasoline.
Calculate how much O2 it consumes and how much CO2
it produces assuming complete fuel combustion. Let us take the gasoline
density to be 730 kg.dm3 and the elemental composition 89 %
C and 11 % H (weight %).
Solution:
C + O2 ® CO2
4 H + O2 ® 2 H2O
Gasoline weight m = 7.0 dm3 . 0.730 kg.dm3 = 5.11
kg
Weight of C m(C) = 0.89 . 5.11 kg
Weight of H m(H) = 0.11 . 5.11 kg
Substance amount of C n(C) = 0.89 . 5.11 kg/0.012 kg.mol-1 =
379 mol
Substance amount of H n (H) = 0.11 . 5.11 kg/0.001 kg.mol-1 =
562 mol
The equations show that n (CO2) = n(C) and n (O2)
= n(C) + 1/4 n (H)
The combustion thus gives rise to 379 mol CO2 and consumes
(379 + 562/4)mol = 519.5 mol O2
Weight of produced CO2 m (CO2) = 379 mol . 0.044
kg.mol-1 = 16.7 kg
Weight of consumed O2 m (O2) = 519.5 mol . 0.032
kg.mol-1 = 16.6 kg
The result was fairly surprising. In the future we shall probably rather
walk or ride a bicycle.
Important for emission abatement is the pressure from state authorities, environmentalists and the public, which results in ever more strict emission limits. The initiative for reducing the environmental impact of motor vehicles originated in USA, the strictest regulations being upheld in California. Japan and Canada do not lag much behind. Western European countries have been introducing stricter regulations with a delay of several years after USA. The countries of Central and Eastern Europe will have to adjust quickly to these regulations.
The legislative pressure is exerted in two directions:
1) pressure on fuel composition and thus on refineries
2) pressure on car manufacturers
Measures adopted by refineries
- lowering of the content of aromatics, especially benzene, for which
a 2 % limit has already been set - but benzene in emissions can arise by
dealkylation of other aromatics, especially toluene and xylenes
- presence of oxygen-containing compounds which lower the emissions
of CO and HC
- lowering of the content of sulphur in Diesel oil
Measures adopted by car manufacturers
- electronic fuel injection units instead of carburettors
- aluminium cylinder heads (NOx production is reduced thanks
to better heat removal)
- changes in the combustion space construction
- catalytic conversion of pre-formed harmful substances to CO2,
H2O, N2
Catalytic converters of exhaust gases
The use of catalytic converters has first become widespread in the
USA (beginning in 1975), then in Canada and Japan. The EU countries began
using converters in the eighties. Beginning on 1 January 1993 they started
to have belong to the standard equipment of all produced cars (including
the Czech Republic). The development of catalytic converters has reached
the stage of three-way controlled converters. These are capable of simultaneously
converting CO, HC and NOx. The following reactions then occur
simultaneously:
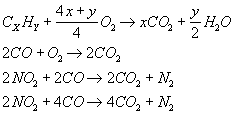
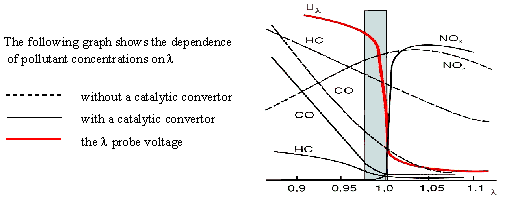
Oxidation reactions proceed the better the higher the oxygen level (for oxygen-poor mixtures), i.e. l > 1. The amount of CO is then insufficient for NOx reduction. On the other hand, in rich mixtures (l < 1) lack of oxygen permits the oxidation of only small amounts of CO and HC but the degree of NOx conversion is high. An optimum degree of conversion of all harmful substances is reached in a narrow range around l = 1.
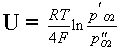
The catalytic converter consists of a ceramic carrier with many channels the surface of which is overlaid with the catalyst. In current use are platinum metals Pt, Rh, Pd (Pt : Rh is usually 1:5) or oxide-based catalysts (NiO, Cr2O3, CuO, MnO2).
Current catalytic converters in an optimum regime can remove harmful substances from 96 - 98 %. A problem is their low efficiency at low temperature (cold starts). This can be alleviated by, e.g., electrical heating of the converter and recycling of combustion products. The conversion ability of the catalyst gradually decreases during operation. If the catalyst is not poisoned or extremely overheated, the manufacturers guarantee a satisfactory efficiency up to 100 000 km.
Platinum metal-based converters can be poisoned above all by lead, phosphorus, sulphur and some construction metals. The main problem in the Czech Republic is lead (the standard for unleaded gasoline allows 0.013 g Pb/l). Phosphorus arrives from lubrication oil additives.